Introduction:
Lithium-ion batteries have revolutionized the world of portable electronics and are increasingly being used in electric vehicles and renewable energy storage systems. Understanding how these batteries work is essential to grasp their advantages and potential applications. In this article, we will provide a comprehensive and user-friendly explanation of the inner workings of lithium-ion batteries.
1.The Basics of Lithium-Ion Batteries:
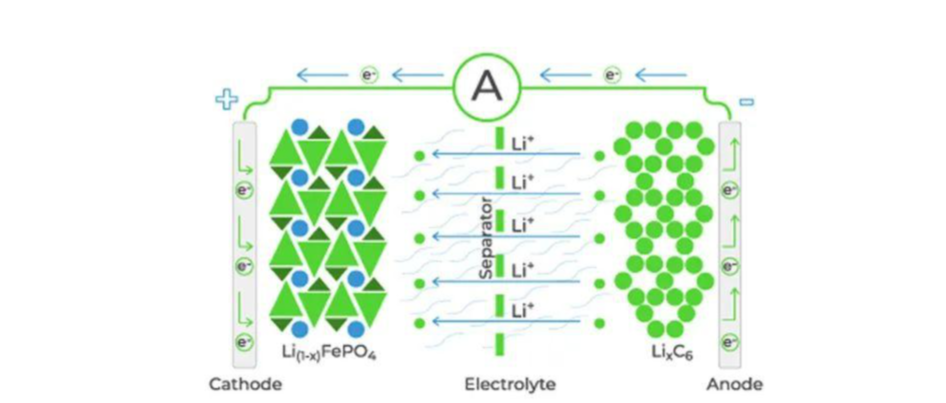
Lithium-ion batteries are rechargeable energy storage devices that utilize lithium ions moving between two electrodes - the cathode and the anode - during charge and discharge cycles. The movement of ions enables the flow of electrical current.
2.Electrolyte:
The battery's electrolyte is a key component that facilitates the movement of lithium ions. It is typically a liquid or gel-like substance composed of lithium salts dissolved in an organic solvent. The electrolyte acts as a medium through which the lithium ions can travel between the electrodes.
3.Cathode:
The cathode is the positive electrode in a lithium-ion battery. It is usually made of a metal oxide compound, such as lithium cobalt oxide (LiCoO2) or lithium iron phosphate (LiFePO4). During discharge, lithium ions move from the cathode to the anode, releasing energy in the process.
4.Anode:
The anode is the negative electrode in a lithium-ion battery and is typically made of graphite or other carbon-based materials. When the battery is charged, lithium ions are extracted from the cathode and inserted into the anode, storing energy.
5.Separators:
A separator is a porous material placed between the cathode and the anode to prevent direct contact, which could cause a short circuit. The separator allows the movement of lithium ions while blocking the flow of electrons.
6.Charging Process:
During the charging process, an external power source applies a voltage higher than the battery's voltage. This causes lithium ions to migrate from the cathode to the anode, where they are stored in the anode's carbon structure.
7.Discharging Process:
When a lithium-ion battery is in use, the stored energy is released through the discharging process. The lithium ions move from the anode to the cathode through the electrolyte, generating an electric current that can power devices or systems.
8.Safety Measures:
Lithium-ion batteries incorporate safety features to prevent overcharging and overheating, which could lead to thermal runaway or even fire. These safety measures include protective circuits and thermal management 9.systems.
Advantages of Lithium-Ion Batteries:
Lithium-ion batteries offer several advantages, including high energy density, lightweight design, no memory effect (no need for full discharge before recharging), and a relatively low self-discharge rate.
Conclusion:
Lithium-ion batteries have become the preferred choice for various applications due to their excellent energy storage capabilities. Understanding the basic principles of how these batteries work, from the movement of lithium ions between the cathode and anode to the role of the electrolyte and safety measures, allows us to appreciate their efficiency and reliability. The continued development of lithium-ion battery technology holds great promise for the future of portable electronics, electric vehicles, and renewable energy storage systems.
